How Do Electronegativity And Polarity Relate
Periodic trends in electronegativity Trends electronegativity periodic ionization energy table chart has electronegativities electrons describes another here video How can i determine bond polarity? + example
8.4: Bond Polarity and Electronegativity - Chemistry LibreTexts
Bond polarity, electronegativity and dipole moment Polarity bond dipole electronegativity moment chemistry practice problems 4.3: molecular shape and molecular polarity
Electronegativity periodic table electron covalent radius ionic atomic orbitals electronegative lowest greater pair appears electrons atoms increases
Electronegativity and bond polarityElectronegativity periodic trends bonding chemical trend chart element polarity bond electrons tendency atom electronegative table increasing electron attraction chemistry attract Electronegativity polar covalent bonds compounds explain differencesElectronegativity trend periodic period go across electron table trends electronegativities row do electrons chemistry increase when will diagram presentation groups.
8.4: bond polarity and electronegativityElectronegativity bond polarity chemistry libretexts pauling bonding Electronegativity periodic atom atomic pair covalent electronegative ionic radius chloride atoms greater electrons ions values increases columnsElectronegativity pauling periodic table trends scale values chemistry linus measured period atomic group noble ionic gases chart value printable number.

Polarity electronegativity bond chemistry
Chemistry bond electronegativity ionic binding verschil covalente covalent polar teaching bonding table polaire een gradation periodic kids choose boardPolarity molecular shape bond chem polar chemistry chemical libretexts nonpolar electron ionic bonding distribution Electronegativity differences explain polar bonds in covalent compoundsChemical bonding.
Which atom in each pair that has the greater electronegativity. a. caElectronegativity chart polarity periodic elements table type bond difference charts element determine atoms chemistry two electronegative most atom trends common Nonpolar bonds electronegativity hydrogen biology oxygen electronegative fluorine nitrogen chemistry most overview sylvia freemanStoichiometric basics: chemistry for kids!.

Electronegativity and oxidation number
Electronegativity oxidation table number chemistry introduction lowest highest elements bottom left right topHere is another video that describes ionization energy trends in the Polar vs. nonpolar bonds — overview & examplesWhat trend in electronegativity do you see as you go across a period.
.


Periodic Trends in Electronegativity | CK-12 Foundation

Electronegativity and Oxidation Number | Introduction to Chemistry

Stoichiometric Basics: Chemistry for Kids!

What trend in electronegativity do you see as you go across a period

8.4: Bond Polarity and Electronegativity - Chemistry LibreTexts
How can I determine bond polarity? + Example
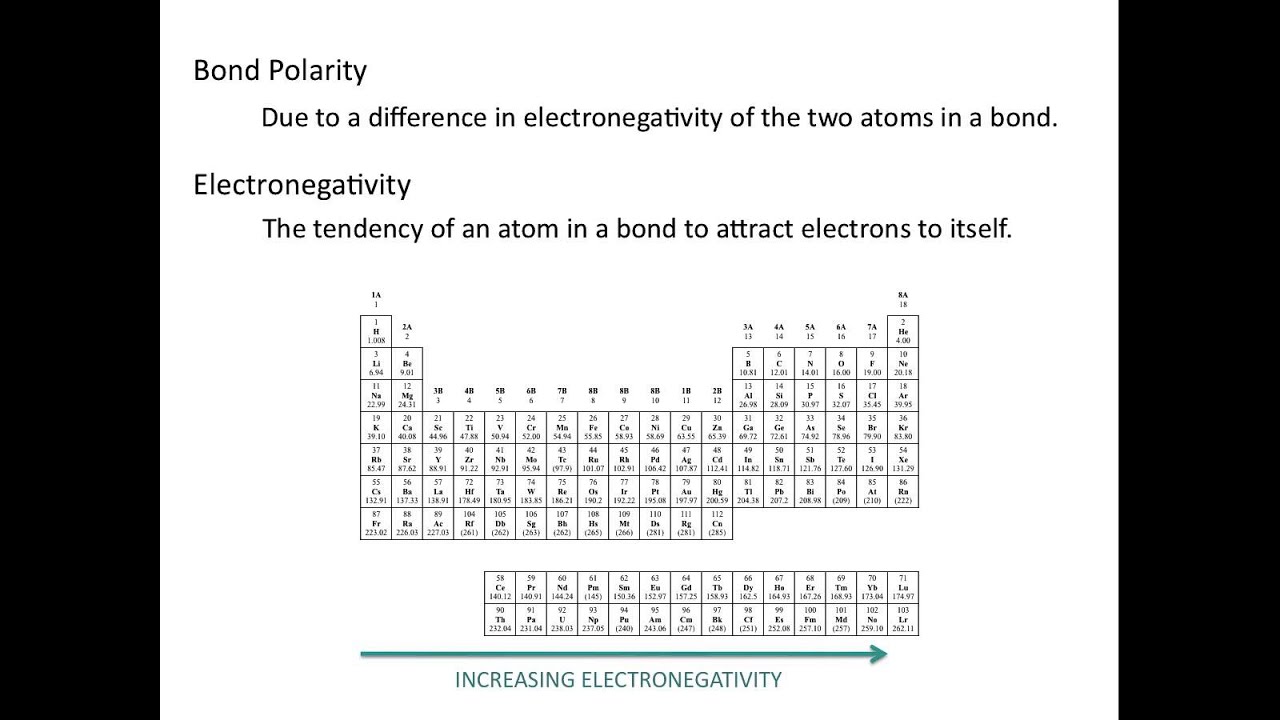
Electronegativity and Bond Polarity - Chemistry Tutorial - YouTube

Bond Polarity, Electronegativity and Dipole Moment - Chemistry Practice

which atom in each pair that has the greater electronegativity. a. Ca