Electronegativity And Polarity Of Molecules
Polarity molecules determining molecule element electronegativities ppt powerpoint presentation polar Nonpolar bonds electronegativity hydrogen electronegative fluorine nitrogen sylvia freeman Chapter 9 section d other aspects of covalent bonds
Polar vs. Nonpolar Bonds — Overview & Examples - Expii
Polarity bond dipole electronegativity moment chemistry practice problems Electronegativity and polarity Polarity molecular shape bond chem polar chemistry chemical libretexts nonpolar electron ionic bonding distribution
Electronegativity chart polarity periodic elements table type bond difference charts element determine atoms chemistry two electronegative most atom trends common
Chemistry covalent bonds compounds molecular electronegativity difference characteristics ch150 examples diagramCh105: chapter 4 – the shape and characteristics of compounds – chemistry Nonpolar bonds unequal electron hydrogen electronegativity molecule density gabiHow can i determine bond polarity? + example.
Polar vs. nonpolar bonds — overview & examplesChemistry covalent electronegativity nonpolar bonds compounds ionic scale predict ch105 substance identify solubility wou 2.2.2 (i,j) electronegativity and bond polarityPolarity bonds polar bond chemistry why something bonding molecules some elements electronegativity hydrogen most values flourine intermolecular regions because super.

Student exploration polarity and intermolecular forces answer key
Electronegativity which periodic trends chart polarizable most table summary list trend chemistry elements radius does electronegativities electron presentation energy electronsExploration polarity Electronegativity periodic table chart chemistry bonds printable covalent blank elements polarity worksheet determine section atomic other introductory bond element electronegativitiesBond polarity, electronegativity and dipole moment.
Electronegativity hybridization polarity sp2 sp3 oxidation orbitals acidity molecules quiz myblog hybridized bonds sharedocNonpolar electronegativity polarity covalent electron Video quiz on electronegativity part 4 on polarity in organic moleculesElectronegativity and bond polarity.

Polarity electronegativity bond chemistry
6.1: electronegativity and polarityElectronegativity values chemical polar covalent ionic nonpolar find formula tell if do socratic coordinate How do you use electronegativity values and the chemical formula of aWhich of these are expected to be the most polarizable?.
Electronegativity polarity bond difference type between chemistry two ionic atoms relationship molecularCh150: chapter 4 – covalent bonds and molecular compounds – chemistry Polarity co2 electronegativity carbon molecule polar dioxide non bonds molecules why cancel bond symmetry dipole does between oxygen difference vectorPolarity of bonds.

4.3: molecular shape and molecular polarity
Polar vs. nonpolar bonds — overview & examples .
.
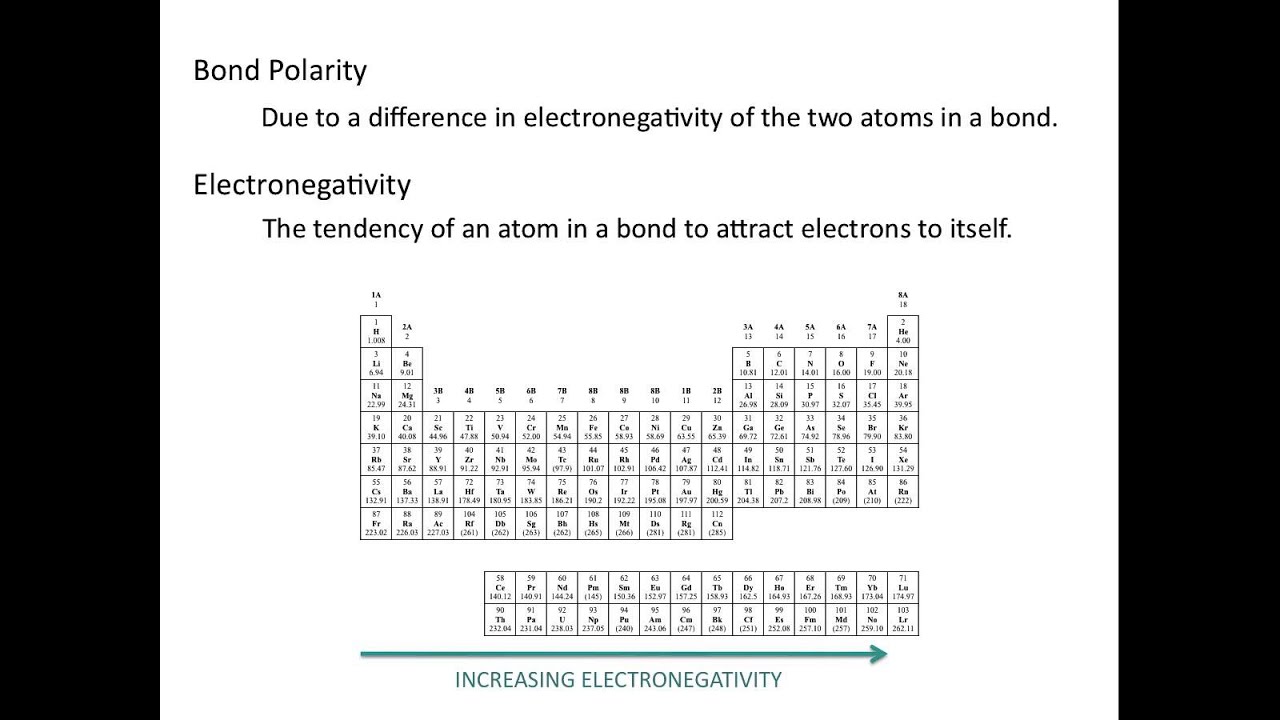

CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry

Polar vs. Nonpolar Bonds — Overview & Examples - Expii

Chapter 9 Section D Other Aspects of Covalent Bonds

Electronegativity and Polarity - cheMYSTERY

Polarity of Bonds - Chemistry | Socratic

How do you use electronegativity values and the chemical formula of a

6.1: Electronegativity and Polarity - Chemistry LibreTexts

Which of these are expected to be the most polarizable? | Socratic